Electrical Engineering ⇒ Topic : Construction of a Lead-acid Battery
|
|
| Sachin
| |
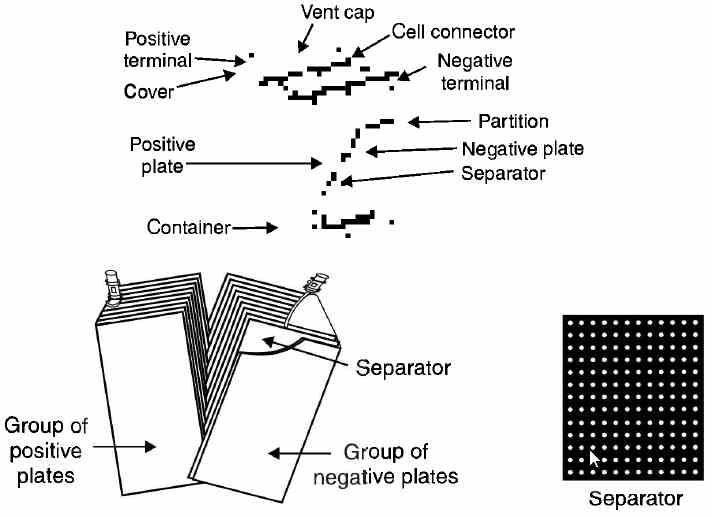
Construction of a Lead-acid Battery A lead-acid battery is a collection of a number of lead-acid cells connected in series; the most common ones being 6-volt type and 12-volt type. In case of 6-volt type, three cells are connected in series whereas for 12-volt type, six cells are series-connected. A commercial lead-acid cell incorporates many refinements in its construction that make it possible to increase its capacity and efficiency. Fig. (a) shows the cut-away view of a commercial lead-acid battery. The various parts are (1) Container. The container houses the plates and the electrolyte. The container of some batteries is made of glass or transparent synthetic plastic material. The main advantage of using these materials is that a visual examination can be made of the state of plates. However, if the battery is used in an automobile, a more robust material (e.g. hard rubber or a strong synthetic plastic material) is required to withstand the mechanical shocks. The container is sealed off at the top to prevent the spilling of electrolyte. A large space is left at the bottom of the container so that any sediment that drops from the plates may collect there without causing the short-circuit between positive and negative plates. (2) Plates. The capacity of a lead-acid cell *depends upon the plate area. To increase the effective area of plates without increasing the size of the cell, we use a large number of thin plates in the cell instead of two plates. The alternate positive and negative plates of the cell are sandwitched together with insulators (called separators). The negative plates are connected together as are the positive plates. A commercial cell always contains an odd number of plates such as 11,13, 15 or 17. The number of negative plates is always one more than the number of positive plates ; the outside plates being negative. This arrangement not only allows both sides of the positive plate to be used actively but also the tendency for one side of the plate to distort is balanced by a similar effect on the other. figure (a) A separate compartment is provided for each cell and each compartment has large space at the bottom so that any sediment from the plates may collect there. In a 6-V battery, three cells are assembled, each in an acid proof compartment, the cells are then connected in series. A final point about the "formation" of plates. The type of plate construction (i.e. Plante plate or Faure plate) to be used will depend upon the service requirements. (3) Separators. In order to save space and to reduce the internal resistance of the cell, the plates are placed close together. To prevent the plates from touching each other if they wrap or buckle, they are separated by non-conducting materials (e.g. wood, rubber etc) called separators. Separators are grooved vertically on one side and are smooth on the other side. The grooved side is placed against the positive plate to permit free circulation of electrolyte around the positive plate where *greater chemical action takes place. (4) Electrolyte. The electrolyte is dilute sulphuric acid (H2S04) solution mixed in such a proportion so that with a fully charged battery, its specific gravity is about 1.28. During the normal life of a battery that is properly cared for, the electrolyte loses none of the acid. It is, therefore, necessary only occasionally to replace the water that has evaporated. To ensure normal battery life, only pure water should be added. (5) Cell cover. Each cell compartment has a cover, usually made of moulded hard rubber. Openings are provided in these covers for the two terminal posts and for a vent. The joints between covers and container are sealed with an acid-resistant material. (6) Vent caps. Each cell cover has a hole into which is fitted the vent cap. This cap has a vent hole to allow the free exit of the gas formed in the cell. The vent caps can be easily removed for adding water or taking hydrometer reading. (7) Inter-cell connector. It is a lead alloy link that joins the cells in series. The positive terminal of the cell is marked by a large + sign or with a red colour. (8) Cell terminals. Each cell has two terminals. The terminals are generally made of lead as it does not con-ode due to the electrolyte | |
|
| |
!! OOPS Login [Click here] is required for more results / answer